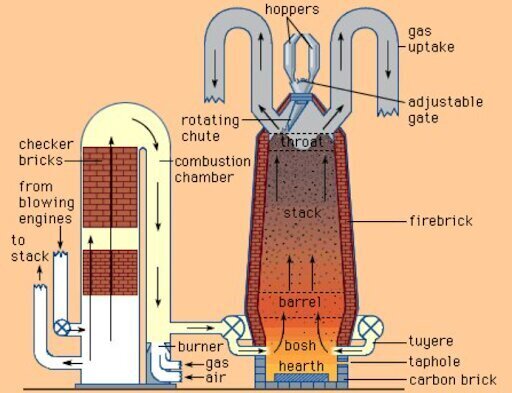
Blast Furnace
Blast furnace, a vertical shaft furnace that produces liquid metals by the reaction of a flow of air introduced under pressure into the bottom of the furnace with a mixture of metallic ore, coke, and flux fed into the top. Blast furnaces are used to produce pig iron from iron ore.
Blast furnaces operate on the principle of chemical reduction whereby carbon monoxide converts iron oxides to elemental iron. Blast furnaces differ from bloomeries and reverberatory furnaces in that in a blast furnace, flue gas is in direct contact with the ore and iron, allowing carbon monoxide to diffuse into the ore and reduce the iron oxide. The blast furnace operates as a countercurrent exchange process whereas a bloomery does not. Another difference is that bloomeries operate as a batch process whereas blast furnaces operate continuously for long periods. Continuous operation is also preferred because blast furnace are difficult to start and stop. Also, the carbon in pig iron lowers the melting point below that of steel or pure iron; in contrast, iron does not melt in a bloomery.
Silica has to be removed from the pig iron. It reacts with calcium oxide (burned limestone) and forms silicates, which float to the surface of the molten pig iron as "slag". Historically, to prevent contamination from sulfur, the best quality iron was produced with charcoal.